Digitizing
This working group has been focused on identifying, increasing awareness of and pursuing opportunities to digitize, and use technology to support, aspects of the COVID-19 evidence synthesis response to facilitate collaboration and capture efficiencies. It is now on stand-by and willing to take on exciting new opportunities to make a difference.
Terms of reference
- Identify (with the help of other working groups) and document the key barriers for different actors in the evidence synthesis ecosystem, determine whether and how these barriers can be addressed by digital solutions, and prioritize work on digital solutions that would add the most value
- Document digital solutions (existing and in development) that can make information retrieval, curation, processing, generating and publishing more efficient
- What is going on (people, projects, portals and systems), and in the case of portals this includes the following details:
- API
- Other export possibilities (e.g., CSV, Excel, RIS)
- Type of study ID used to identify unique records (e.g., medRxiv, NCTID, PMID, local ID)
- Standardized terminologies used to describe or categorize content (e.g., ACT, ICD-10, MedDRA, MeSH, RxNorm, SNOMED CT)
- Standards (including standardized terminologies), tips and tools available to optimize digital solutions
- What is going on (people, projects, portals and systems), and in the case of portals this includes the following details:
- Add the portals to COVID-END’s guide to COVID-19 evidence sources (if any are not listed now), the standardized terminologies to the COVID-END taxonomy, and the digitization tips and tools to ‘tips and tools’ part of the COVID-END website
- Identify gaps in digital solutions and standards (to spur their development or funding to support development) and describe work underway on digital solutions and standards (to spur collaboration)
- Prioritize key gaps in standards that should be addressed as soon as possible and describe what could be done better or more efficiently if the evidence synthesis ecosystem had more such standards in place (to spur their implementation or funding to support implementation)
Participants
- Chris Mavergames, Cochrane Collaboration, Germany (co-chair)
- Linn Brandt, Magic Evidence Ecosystem Foundation, Norway (co-chair)
- Alfonso Iorio, McMaster University, Canada
- Brian Alper, EBSCO, United States
- Gabriel Rada, Epistemonikos, Chile
- Gordon Dooley, Centre for Research and Dissemination, PROSPERO (University of York), UK
- Gunn Vist, Norwegian Institute of Public Health, Norway
- James Thomas, EPPI Centre, UK
- Jerry Osheroff, ACTS, U.S.A.
- Jon Brassey, TRIP database, UK
- Julian Elliott, Cochrane Australia, Australia
- Tamara Navarro, McMaster University, Canada
- Secretariat: Kaelan Moat, McMaster Health Forum and COVID-END Secretariat, Canada; David Tovey, COVID-END Secretariat
Meeting documents
| Meeting date | Documents |
| November 6, 2020 | |
| October 9, 2020 | |
| September 25, 2020 | |
| September 18, 2020 | |
| September 11, 2020 | |
| August 28, 2020 | |
| August 21, 2020 | |
| August 14, 2020 | |
| July 24, 2020 | |
| July 17, 2020 | |
| July 10, 2020 | |
| June 26, 2020 | |
| June 19, 2020 | |
| June 12, 2020 | |
| June 5, 2020 | |
| May 29, 2020 | |
| May 22, 2020 | |
| May 15, 2020 | |
| May 8, 2020 | |
| April 30, 2020 |
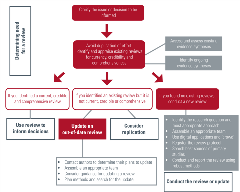
Use the interactive flow diagram to find resources for researchers considering and conducting COVID-19 evidence syntheses.
View the interactive flow diagram