Development, adoption & adaptation
There are various approaches to developing trustworthy CPGs or HTAs depending on the setting, the experience of the developers, and the available resources. We provide a brief overview and linked resources to three primary approaches, bearing in mind that the concept of living CPGs adds opportunities and challenges to traditional ways of authoring, publishing and updating recommendations for policy and practice.
De Novo development
De novo (new) guideline development should be considered when there are no up-to-date, high quality and relevant guidelines that answer the question(s) at hand, or when there is only out-of-date and/or poor quality guidance available. There are various approaches to developing guidelines de novo; however, the fundamental steps remain similar. The below figure depicts the typical de novo CPG process, also applicable to HTA development. The direction of the guideline or HTA process starts at the top at priority setting and continues down to updating the guidelines.
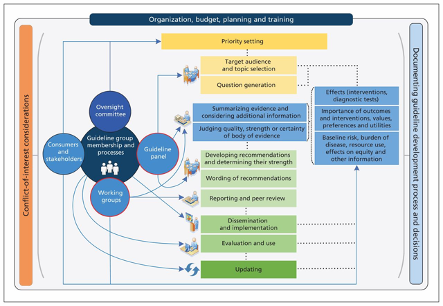
*See Schünemann et al. (2014) for details.
Some resources for developing CPGs and HTAs de novo:
- World Health Organization Handbook for Guideline Development
- Clinical Practice Guidelines we can trust (Institute of Medicine, US)
- Guidelines 2.0 (GIN-McMaster checklist)
- GIN Standards for Clinical Practice Guidelines
- GIN-McMaster guideline development checklist for rapid recommendations
- American College of Physicians Methods for Developing Clinical Practice Guidelines
- NICE Guideline development manual
- NHMRC Guidelines for Guidelines
- HTAi Vortal
Methods for assessing the quality of the synthesised evidence
Currently, the most widely accepted and adopted approach to assessing the quality (also called certainty) of the synthesised evidence is the GRADE approach. GRADE (Grading of Recommendations, Assessment, Development and Evaluations) is a transparent framework for developing and presenting summaries of evidence and provides a systematic approach for developing guideline recommendations.
For assessing the quality (or certainty) of the body of evidence, GRADE considers a series of criteria to rate the evidence (including when to upgrade or downgrade) and creates evidence tables that summarise the final quality assessment, per outcome of interest. The 5 criteria advising downgrading the quality (or certainty) of randomised controlled trials (RCTs) are study limitations (risk of bias), publication bias, imprecision, inconsistency and indirectness, and the 3 criteria for potentially upgrading observational evidence are, large magnitude of effect, dose-response gradient, and effect of plausible residual confounding. The GRADE handbook provides more information on how to apply the GRADE approach. When doing qualitative evidence syntheses, GRADE-CerQUAL should be used. Overall quality (or certainty) of evidence (per outcome) is stated as either High, Moderate, Low or Very Low, depending on the grading assessment.
For developing recommendations, the GRADE working group recommends the use of the Evidence-to-Decision framework (EtD). The EtD is an explicit and transparent framework for decision-making that can help to ensure that all important criteria are considered and that decisions are informed by the best available research evidence. EtD considers the following domains: priority of the problem, certainty of the evidence, outcome importance, balance between desirable and undesirable effects, resource use, equity, acceptability, and feasibility. An alternative EtD framework has been recommended by the WHO to include factors that may have been neglected and align better with WHO resources scope, in the WHO-INTEGRATE framework. This framework is applicable to any evidence-based guidance document but may be better suited for decisions about population-level and system-level interventions at the global as well as national levels.
Alternative guideline development methods (Guideline adoption or adaption)
De novo guideline development is often out of reach for resource constrained guideline development teams who may lack the funding and time needed for de novo methods. In this case, existing high-quality (e.g. assessed via AGREE II), relevant and up-to-date guidelines can be adopted or adapted for local use.
Guideline adoption
Adopting a guideline means to select a guideline (typically the most trustworthy, up-to-date and relevant for a specific context), and use or implement it in the context of interest. Guidelines or recommendations can be considered for adoption if there is no need to change the recommendation, the evidence base, or how it is implemented in a local setting; considering factors such as cost, workforce, health systems, management options and access to care.
Guideline adaptation
Although some decision makers and organisations would like to have their own guidelines or their own set of recommendations, it is neither efficient nor a responsible allocation of limited resources to develop new guidelines “from scratch” when existing guidelines can be adopted or adapted to meet the organisation’s needs. With the advancement of the methods for developing guidelines in the last decade, in some cases it is very feasible to adapt an existing high-quality CPG to a specific context and obtain high-quality recommendations that are contextually specific. Below, we provide some ideas on what factors should be considered before making a decision about how to adapt evidence-based guidelines, and we propose an algorithm that may be followed to facilitate this decision.
Adaptation is defined by the Guidelines International Network (GIN) as the systematic approach to the modification of a guideline(s) or recommendation(s) produced in one cultural and organisational setting for application in a different context. Adaptation may be used as an alternative to de novo guideline development (e.g., for customising (an) existing guideline/s to suit the local context). Guideline adaptation is more common than guideline adoption.
When selecting the best approach for a specific context, development, group, country or clinical scenario, it is advisable to consider the following factors: capacity, costs, contexts and availability and quality of guidelines.
- ● Capacity: This refers to the ability of the guideline development group to execute a particular guideline development process or method, considering available time, scope and expertise. For example, if the guideline development group lacks the appropriate expertise or human resource capacity to conduct a de novo guideline development project, then alternative guideline development methods such as guideline adoption or adaptation might be preferable.
- Costs: This refers to the monetary and human resource cost of a particular guideline development approach. For example, if the guideline development group lacks appropriate funding for conducting new or updating systematic reviews as part of a de novo development process, then more cost effective approaches should be considered.
- Context: This refers to the various local, national or regional factors that need to be considered when deciding on a guideline development approach. These might include specific local preferences and sensitivities, competing demands, or specific resources (human and otherwise).
- Availability and quality of guidelines: Guidelines adaptation or adoption methods are dependent on a current, high-quality pool of guidelines. If, for a particular topic, no available guidelines exist, then de novo guideline development may be the only option. Alternatively, if guideline quality is lacking or out of date, then guideline adaption or updating should be considered. Adaptation processes heavily depend on the quality of the guidelines and evidence syntheses that are available.
We suggest considering the above factors when choosing best approach to the specific context and scenario. More information on different approaches to adapt guidelines are detailed below:
- GRADE-ADOLOPMENT approach
- ADAPTE Toolkit
- RAPADAPTE approach
- PAHO approach
- Adapt, Adopt and Contextualise (SAGE)
- American College of Physicians Guidance Statements Approach using AGREE Instrument
For further examples and resources for alternative guideline development methods, see the examples and resources of guideline adaptation from LMICs.
